Saucerman, Brunton, Michailova, McCulloch, 2003
Model Status
This CellML version of the Saucerman and McCulloch 2003 model is a translation of Jeff Saucerman's original MATLAB code, where all figures of the original paper are reproduced. In this CellML version, units are consistent.
Model Structure
ABSTRACT: The beta-adrenergic signaling pathway regulates cardiac myocyte contractility through a combination of feedforward and feedback mechanisms. We used systems analysis to investigate how the components and topology of this signaling network permit neurohormonal control of excitation-contraction coupling in the rat ventricular myocyte. A kinetic model integrating beta-adrenergic signaling with excitation-contraction coupling was formulated, and each subsystem was validated with independent biochemical and physiological measurements. Model analysis was used to investigate quantitatively the effects of specific molecular perturbations. 3-Fold overexpression of adenylyl cyclase in the model allowed an 85% higher rate of cyclic AMP synthesis than an equivalent overexpression of beta 1-adrenergic receptor, and manipulating the affinity of Gs alpha for adenylyl cyclase was a more potent regulator of cyclic AMP production. The model predicted that less than 40% of adenylyl cyclase molecules may be stimulated under maximal receptor activation, and an experimental protocol is suggested for validating this prediction. The model also predicted that the endogenous heat-stable protein kinase inhibitor may enhance basal cyclic AMP buffering by 68% and increasing the apparent Hill coefficient of protein kinase A activation from 1.0 to 2.0. Finally, phosphorylation of the L-type calcium channel and phospholamban were found sufficient to predict the dominant changes in myocyte contractility, including a 2.6x increase in systolic calcium (inotropy) and a 28% decrease in calcium half-relaxation time (lusitropy). By performing systems analysis, the consequences of molecular perturbations in the beta-adrenergic signaling network may be understood within the context of integrative cellular physiology.
The original paper reference is cited below:
Modeling beta-adrenergic control of cardiac myocyte contractility in silico , Jeffrey J. Saucerman, Laurence L. Brunton, Anushka P. Michailova, and Andrew D. McCulloch, 2003, Journal of Biological Chemistry, 48, 47997-48003. PubMed ID: 12972422
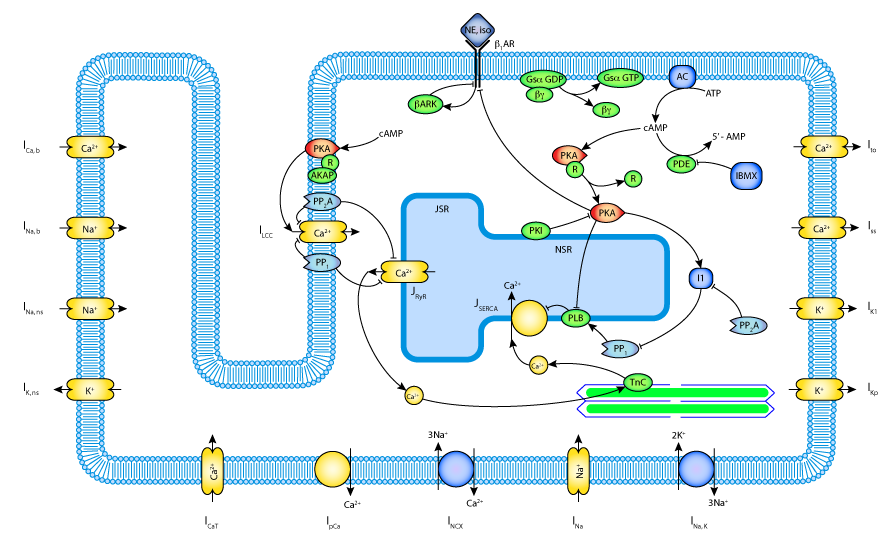 |
| Schematic diagram of the integrated model components, including the beta-adrenergic network, calcium handling, and the electrophysiology of the rat ventricular myocyte. |
