Computational modeling reveals key contributions of KCNQ and hERG currents to the malleability of uterine action potentials underpinning labor
Model Status
This CellML model is based on a translation of the original C code. The equations are validated using OpenCell and all units are consistent. The model can be run in both OpenCell (version 0.8) and OpenCOR (version 0.4) environments using any of the integration methods. To recreate the published results, for example figure 7 from the original paper, apply a suitable stimulating current and integrate the model for a total 60 sec with a time step of 0.02 ms.
Model Structure
ABSTRACT: The electrical excitability of uterine smooth muscle cells is a key determinant of the contraction of the organ during labor and is manifested by spontaneous, periodic action potentials (APs). Near the end of term, APs vary in shape and size reflecting an ability to change the frequency, duration and amplitude of uterine contractions. A recent mathematical model (PubMed ID: 21559514, CellML model) quantified several ionic features of the electrical excitability in uterine smooth muscle cells. It replicated many of the experimentally recorded uterine AP configurations but its limitations were evident when trying to simulate the long-duration bursting APs characteristic of labor. A computational parameter search suggested that delayed rectifying K+ currents could be a key model component requiring improvement to produce the longer-lasting bursting APs. Of the delayed rectifying K+ currents family it is of interest that KCNQ and hERG channels have been reported to be gestationally regulated in the uterus. These currents exhibit features similar to the broadly defined uterine IK1 of the original mathematical model. We thus formulated new quantitative descriptions for several IKCNQ and IhERG. Incorporation of these currents into the uterine cell model enabled simulations of the long-lasting bursting APs. Moreover, we used this modified model to simulate the effects of different contributions of IKCNQ and IhERG on AP form. Our findings suggest that the alterations in expression of hERG and KCNQ channels can potentially provide a mechanism for fine tuning of AP forms that lends a malleability for changing between plateau-like and long-lasting bursting-type APs as uterine cells prepare for parturition.
The original paper reference is cited below:
Computational modeling reveals key contributions of KCNQ and hERG currents to the malleability of uterine action potentials underpinning labor. Wing-Chiu Tong, Rachel M. Tribe, Roger Smith and Michael J. Taggart, 2014, PLoS One, 9, e114034. PubMed ID: 25474527
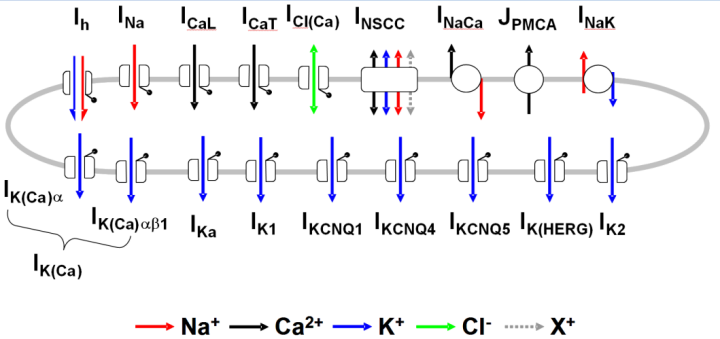 |
| A schematic diagram of the Tong et. al. 2014 uterine smooth muscle cell model. |
